|
Vitamin B3 (Nicotinic acid)
|
Vitamin B3, also known as niacin/nicotinic acid can be synthesized by the body from tryptophan.
The nickname niacin came from linking several letters in the words nicotinic,
acid and vitamin.
As the story goes, when the properties of nicotinic acid (obtained through
the oxidation of nicotine) were first discovered it was wisely decided that vitamin B3 or
nicotinic acid should be named in such a way as to dissociate it from nicotine and not to create
the impression that either smoking provided this vitamin or that wholesome food contained a poison.
Niacin is required for processes such as cell respiration, synthesis of ATP, carbohydrate,
fat and protein metabolism. Approximately 60 mg of tryptophan are necessary for the synthesis
of 1 mg of niacin, which requires the presence of vitamins B6 and B2.
|
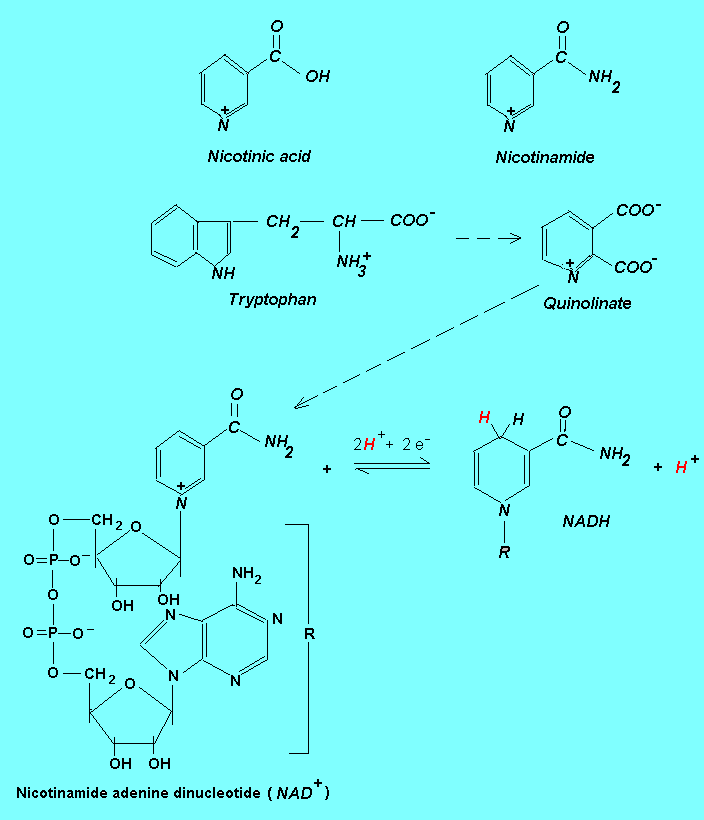
The figure above shows the chemical structure of vitamin B3, the precursor (tryptophan) in the biosynthesis of NAD+ coenzyme and the redox cycling of this coenzyme. In a dehydrogenation reaction where the substrate is oxidized, the oxidized form of the coenzyme (NAD+) accepts a hydride ion (:H-) and becomes reduced. The second hydrogen from the substrate is released to the aqueous solution as a proton. Note that the nitrogen atom in the benzenoid ring of the nicotinamide moiety has a fixed positive charge but the overall charge of both NAD+ and NADP+ is negative (provided by the O- of the phosphate groups). Therefore, the positive sign
in NAD+ and NADP+ does not indicate a positive charge. Rather, it indicates
that the coenzymes are in the oxidized form. Because the NADH/NADPH absorbs light at 340 nm, the
dehydrogenation reactions catalyzed by NAD-dependend dehydrogenases are easily monitored by recording the change in absorption at 340 nm.
|
- Nicotinic acid (as nicotinamide) is part of the chemical structure of the coenzymes NAD and NADP.
The NAD/NADP-dependent enzymes are involved in oxidation-reduction reactions in energy production,
cholesterol metabolism, fatty acid oxidation, glucose degradation and pentose phosphate pathway,
amino acids synthesis and degradation, defence against pathogenic bacteria (NADPH-dependent generation
of oxygen reactive species and the regeneration of GSH from GSSG) and synthesis of glucocorticoid
and sex hormones.
- NAD+ acts as a substrate for many ADP-ribosylation reactions such as mono- and poly ADP-ribosylation of proteins/enzymes, formation of cyclic ADP-ribose and the generation of O-acetyl-ADP-ribose in
deacetylation reactions. Ribosylation normally involves the arginine, cysteine and glutamine residues in proteins.
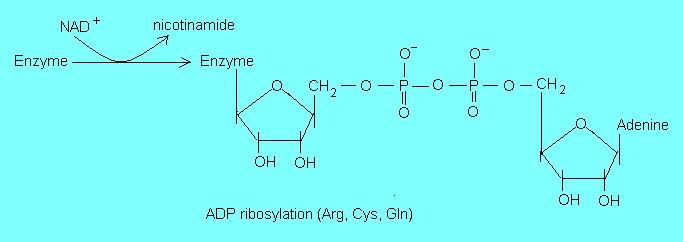
These non-redox reactions are critical in the regulation of cellular metabolism and they are sensitive to dietary niacin status (1). A cellular component that is directly regulated through ADP-ribosylation reactions is chromatin. An altered chromatin function in response to a low niacin status may have far reaching consequences that can affect genomic stability, cell division, differentiation and apoptosis.
- It is important to distinguish between the biochemical role played by the reduced
forms of the coenzymes NAD and NADP, namely NADH and NADPH, respectively. Although these coenzymes
differ chemically only by a phosphate group, they are not metabolically interchangeable because
of the high degree of specificity of the NADH/NADPH-dependent enzymes towards their respective
coenzymes. Thus, NADH is involved heavily in oxidative processes such as oxidative phosphorylation
(ATP synthesis). In such processes the metabolite oxidation is favored by an intracellular
NAD+/NADH ratio of around 1000. In contrast, NADPH uses the free energy of metabolite
oxidation in endergonic reductive biochemical reactions such as NADPH-dependent
reactions in the cholesterol and fatty acid metabolism. In metabolite reduction reactions
the NADP+/NADPH ratio is kept around 0.01.
- Nicotinic acid is produced by the human body from the amino acid tryptophan. However, when
the intake of tryptophan-rich foods (turkey, chicken, fish, cottage cheese, bananas, eggs, nuts,
wheat germ, milk, beans, peas, soya, etc.) is low, the synthesis of NAD and NADP coenzymes is impaired
because all available tryptophan is used in protein synthesis. As a result, vitamin B3 deficiency
may develop.
- Symptoms associated with low intake of tryptophan-rich foods: irritability, nervousness,
fatigue, headaches, loss of appetite, insomnia, skin problems.
- Interactions: Vitamin B3 is inactivated by alcohol and certain drugs.
- Health benefits: supplementation is useful for helping reduce
the high cholesterol blood levels and arthritis/osteoarthritis symptoms.
- Best food sources (also rich in tryptophan): liver and other organ
meats, eggs, fish, peanuts, leaf vegetables, broccoli, tomatoes, carrots, whole grains, milk.
|